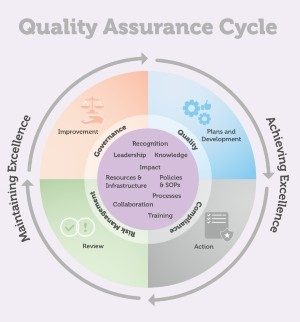
- Implement Quality frameworks and tools to establish and maintain consistent standards and processes across all research projects
- Support adherence to The George Institute India, local and international regulations, policies, guideline and protocols as a way of compliance across all activities
- Strengthen the capacity of researchers and teams to proactively identify risk and control impact to manage project-related risks
- Provide oversight to quality, risk and compliance with ongoing improvements as part of institutional governance.

CORE India - Centre for Operational and Research Excellence
Our Mission: To provide high-quality expertise as well as robust operations, systems and processes to deliver The George Institute’s research strategy
- Scoping funding opportunities for grant proposals and supporting competitive application development
- Coordinating Internal Peer review process and post-award due diligence
- Obtaining regulatory clearances for institutions such as DSIR renewal; organizing institutional ethics review meetings
- Keeping institutional data up to date on grant applications and research publications
- Preparing institution-wide research reports for multiple purposes
- Support data management plan development
- Support development of the database for various study designs using different electronic data-capturing platforms
- Providing budget estimations for different components of project data management
- Help with CRF/Questionnaire designing
- Training researchers on data management and developing data management manuals, SOPs and tools
- Assist with grant applications - study design, sample size, and statistical analysis
- Support sample size calculation, sampling design, randomization, development of analysis plan and statistical analyses
- Training researchers on basic statistical concepts
- Assist investigators and study team with resources (staff/technology) for managing trial operations
- Support the development of operational project activity plan powered on a clinical trials management system -Connect chief investigator and the team with the potential sites based on participant population, intervention, and outcomes.
- Guide project teams in identifying regulatory consultants and vendors for drugs/investigational medicinal products and other consumables required for the study operations.
Quality assurance